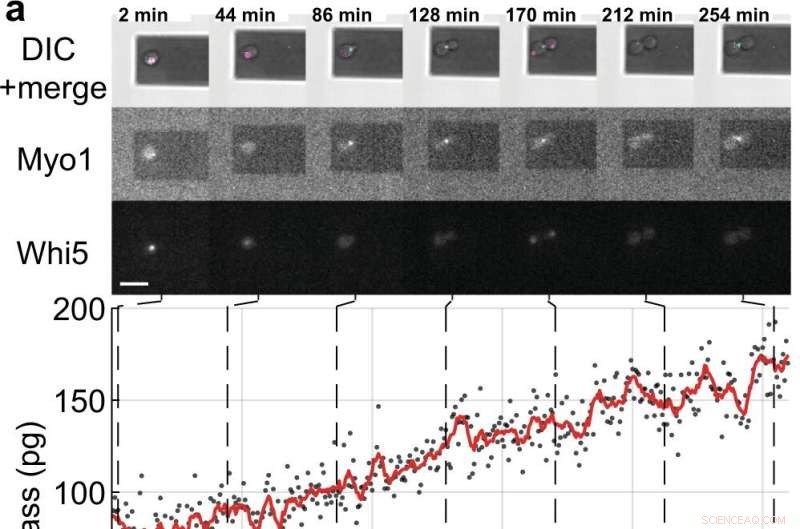
Mass- och cellcykelmätningar av enstaka S. cerevisiae-celler som spirar dotterceller. a, b Enstaka jästceller som uttrycker de fluorescensmärkta cellcykelmarkörproteinerna (Myo1-mKate2 (3×) och Whi5-mKOκ (1×)), avbildades med hjälp av differentiell interferenskontrast (DIC) och fluorescensmikroskopi var 2 min (övre paneler ). En fas- och amplitudkurva för mikrokantilen registrerades över intervaller ≈50 s för att mäta cellmassan med hjälp av svepläget (tilläggsfilm 4). Mellan på varandra följande massmätningar stängdes de infraröda och blå lasrarna av pikobalansen av i ≈ 20 s för att minska blekning av fluoroforerna och för att minska potentiell störning av jästtillväxt. Cellmassavärden härledda från uppsättningar av enstaka amplitudkurvor visas som grå punkter. Genomsnittlig rådata (350 s rörligt fönster, röd linje) visar trenden. Cyanstaplar på tidsaxeln anger S/G2/M-fasen av jästcellcykeln, och magentastaplar anger G1-fasen. Stjärnan (*) i b anger dottercellens (partiella) lossning efter cytokines, vilket sänker den totala massan. Skalstaplar (vita), 10 µm. c Tillväxtkurvor för (n =19) enstaka jästceller som fortskrider genom S/G2/M-fasen (knopptillväxt) mätt med pikobalansen med användning av svepläget i (n =19) oberoende experiment. Den totala tillväxthastigheten mellan start- och slutmassan varierar mellan 0,1 och 2,0 pg min –1 , med ett genomsnitt på 0,7 ± 0,5 pg min –1 (medelvärde ± SD). Varaktigheten av S/G2/M-fasen sträcker sig från 57 till 184 min, med ett genomsnitt på 96 ± 35 min. Kredit:Nature Communications (2022). DOI:10.1038/s41467-022-30781-y
Celler, de mest grundläggande enheterna i livet som bildar alla levande organismer, har länge bevakat sina hemligheter, men nu har ett internationellt team från University of Sydney, ETH Zürich och University of Basel avslöjat några av deras hemligheter genom utvecklingen av en värld -första tekniken.
Forskare vet att celler växer men man trodde att de växer linjärt eller exponentiellt i storlek innan de delar sig.
Nu, i en tidning publicerad i Nature Communications co-led by University of Sydney physicist Dr. David Martinez-Martin, using a nanotechnology technique called "inertial picobalance," scientists have identified that at the single cell level, yeast grow in sequential intervals or segments of linear growth (constant growth rate). At each interval, yeast cells switch to faster or slower growth—a "gear-like" tendency.
The research was performed with saccharomyces cerevisiae, a unicellular yeast organism fundamental in the production of bread, beer, wine and pharmaceuticals. The protein-encoding genes of many types of yeast mirror genes in animal cells, making its behavior key to understanding human disease.
Notably, the behavior found in yeast differs significantly from that of animal cells (including human). It was not until 2017 that Dr. Martinez-Martin and colleagues, also using picobalance, first observed that the mass of living mammalian cells fluctuate intrinsically—they "yo-yo" in size.
"We have uncovered processes that challenge models in biology that have been central for decades," said Dr. Martinez-Martin. "The behaviors we have identified in cells from fungus and animal kingdoms provide strong evidence that cells have different strategies to regulate their mass and size, paving the way to better understand how they can accurately form and reform complex structures such as the eyes, brain and fingers in our bodies."
A recent mathematical model published in Journal of Biological Research—Thessaloniki by Dr. Martinez-Martin also offers fresh insight into the meaning of this once-secretive cellular flux.
"Another of our recent studies has found that while cell mass fluctuations have been detected in single mammalian cells, they can be perfectly viable in organisms comprised of many mammalian cells, including humans. Our modeling suggests that the body's cells don't all swell and decrease at the same time—instead they give and take from each other, maintaining an adequate distribution of the body's mass and volume.
"Mass fluctuations may be used by cells to regulate cellular functions such as metabolism, gene expression, proliferation and cell death, by means of altering the concentration and crowdedness of chemical cellular components."
The model also suggests that mass fluctuations allow cells to communicate, both by acting as biomechanical signals through volume fluctuations, and through the exchange of water and molecules.
"I believe this could be a fundamental mechanism which may help cells locate and communicate their position within an organism," Dr. Martinez-Martin said. "Therefore, it could be incredibly important, because it could allow cells to identify and serve their distinct role and purpose in the body."
"Researchers believe that a better understanding of how cells change their mass and size over time, as well as dysregulation of this process (when cells change their size atypically), could be the key to developing the next generation of diagnostics and treatments for a range of diseases, such as cancer, diabetes and cardiovascular disease."
About inertial picobalance:The technique used in the discovery
Dr. Martinez-Martin, who has been recently distinguished by the World Intellectual Property Organization as a young change maker, is the principal inventor of inertial picobalance, a new technology that measures the mass of single or multiple living cells in real-time, enhancing the understanding of cell physiology. The technology is currently being commercialized by the Swiss nanotech company, Nanosurf AG.
In a Nature paper published in 2017, using inertial picoblance, Dr. Martinez-Martin and his colleagues discovered that the mass of living mammalian cells fluctuates intrinsically by one to four percent over seconds, largely due to water entering and exiting cells.
Using this technique, they were also able to observe cells infected with the vaccinia virus (a virus from the poxvirus family). The infected cells showed different mass behavior over time than non-infected cells, potentially enabling a new way of detecting viral infections. + Explore further