
Färgen på en suspension av nanocages beror på tjockleken på burarnas väggar och storleken på porerna i dessa väggar. Som deras färg, deras förmåga att absorbera ljus och omvandla det till värme kan kontrolleras exakt. Kredit:WUSTL
I en föreläsning han höll 1906, den tyske läkaren Paul Ehrlich myntade termen Zuberkugel, eller "magisk kula, "som stenografi för en mycket riktad medicinsk behandling.
Magiska kulor, även kallade silverkulor, på grund av den folkloristiska tron att endast silverkulor kan döda övernaturliga varelser, förblir målet för läkemedelsutvecklingsarbetet idag.
Ett team av forskare vid Washington University i St. Louis arbetar för närvarande på en magisk kula för cancer, en sjukdom vars behandlingar är notoriskt urskillningslösa och ospecifika. Men deras kulor är guld snarare än silver. Bokstavligen.
Guldkulorna är guldnanocages som, när det injiceras, selektivt ackumuleras i tumörer. När tumörerna senare badas i laserljus, den omgivande vävnaden är knappt uppvärmd, men nanocages omvandlar ljus till värme, dödar de maligna cellerna.
I en artikel som just publicerats i tidskriften Små , teamet beskriver den framgångsrika fototermiska behandlingen av tumörer hos möss.
Teamet inkluderar Younan Xia, Ph.D., James M. McKelvey professor i biomedicinsk teknik vid School of Engineering and Applied Science, Michael J. Welch, Ph.D., professor i radiologi och utvecklingsbiologi vid School of Medicine, Jingyi Chen, Ph.D., forskarassistent i biomedicinsk teknik och Charles Glaus, Ph.D., en postdoktor vid avdelningen för radiologi.
"Vi såg betydande förändringar i tumörmetabolism och histologi, " säger Welch, "vilket är anmärkningsvärt med tanke på att arbetet var utforskande, laserns "dos" hade inte maximerats, och tumörerna var "passivt" snarare än "aktivt" riktade."
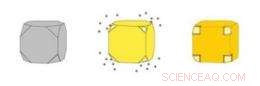
Guld nanocages (höger) är ihåliga lådor gjorda genom att fälla ut guld på silver nanokuber (vänster). Silvret eroderar samtidigt inifrån kuben, kommer in i lösningen genom porer som öppnar sig i kubens avklippta hörn. Kredit:WUSTL
Varför nanocages blir varma
Själva nanocages är ofarliga. "Guldsalter och guldkolloider har använts för att behandla artrit i mer än 100 år, " säger Welch. "Folk vet vad guld gör i kroppen och det är inert, så vi hoppas att detta kommer att vara ett giftfritt tillvägagångssätt."
"Nyckeln till fototermisk terapi, säger Xia, "är burarnas förmåga att effektivt absorbera ljus och omvandla det till värme."
Suspensioner av guld nanocages, som är ungefär lika stora som en viruspartikel, är inte alltid gula, som man kan förvänta sig, men kan istället vara vilken färg som helst i regnbågen.
De är färgade av något som kallas ytplasmonresonans. En del av elektronerna i guldet är inte förankrade till enskilda atomer utan bildar istället en fritt flytande elektrongas, Xia förklarar. Ljus som faller på dessa elektroner kan driva dem att svänga som en. Denna kollektiva svängning, ytplasmon, väljer en viss våglängd, eller färg, ut ur det infallande ljuset, och detta avgör vilken färg vi ser.
Medeltida hantverkare gjorde rubinrött målat glas genom att blanda guldklorid i smält glas, en process som lämnade små guldpartiklar suspenderade i glaset, säger Xia.
The resonance — and the color — can be tuned over a wide range of wavelengths by altering the thickness of the cages' walls. För biomedicinska tillämpningar, Xia's lab tunes the cages to 800 nanometers, a wavelength that falls in a window of tissue transparency that lies between 750 and 900 nanometers, in the near-infrared part of the spectrum.
Light in this sweet spot can penetrate as deep as several inches in the body (either from the skin or the interior of the gastrointestinal tract or other organ systems).
The conversion of light to heat arises from the same physical effect as the color. The resonance has two parts. At the resonant frequency, light is typically both scattered off the cages and absorbed by them.
By controlling the cages' size, Xia's lab tailors them to achieve maximum absorption.
Passive targeting
"If we put bare nanoparticles into your body, " says Xia, "proteins would deposit on the particles, and they would be captured by the immune system and dragged out of the bloodstream into the liver or spleen."
För att förhindra detta, the lab coated the nanocages with a layer of PEG, a nontoxic chemical most people have encountered in the form of the laxatives GoLyTELY or MiraLAX. PEG resists the adsorption of proteins, in effect disguising the nanoparticles so that the immune system cannot recognize them.
Instead of being swept from the bloodstream, the disguised particles circulate long enough to accumulate in tumors.
A growing tumor must develop its own blood supply to prevent its core from being starved of oxygen and nutrients. But tumor vessels are as aberrant as tumor cells. They have irregular diameters and abnormal branching patterns, but most importantly, they have thin, leaky walls.
The cells that line a tumor's blood vessel, normally packed so tightly they form a waterproof barrier, are disorganized and irregularly shaped, and there are gaps between them.
The nanocages infiltrate through those gaps efficiently enough that they turn the surface of the normally pinkish tumor black.
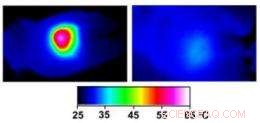
Infrared images made while tumors were irradiated with a laser show that in nanocage-injected mice (left), the surface of the tumor quickly became hot enough to kill cells. In buffer-injected mice (right), the temperature barely budged. This specificity is what makes photothermal therapy so attractive as a cancer therapy. Kredit:WUSTL
A trial run
In Welch's lab, mice bearing tumors on both flanks were randomly divided into two groups. The mice in one group were injected with the PEG-coated nanocages and those in the other with buffer solution. Several days later the right tumor of each animal was exposed to a diode laser for 10 minutes.
The team employed several different noninvasive imaging techniques to follow the effects of the therapy. (Welch is head of the oncologic imaging research program at the Siteman Cancer Center of Washington University School of Medicine and Barnes-Jewish Hospital and has worked on imaging agents and techniques for many years.)
During irradiation, thermal images of the mice were made with an infrared camera. As is true of cells in other animals that automatically regulate their body temperature, mouse cells function optimally only if the mouse's body temperature remains between 36.5 and 37.5 degrees Celsius (98 to 101 degrees Fahrenheit).
At temperatures above 42 degrees Celsius (107 degrees Fahrenheit) the cells begin to die as the proteins whose proper functioning maintains them begin to unfold.
In the nanocage-injected mice, the skin surface temperature increased rapidly from 32 degrees Celsius to 54 degrees C (129 degrees F).
In the buffer-injected mice, dock, the surface temperature remained below 37 degrees Celsius (98.6 degrees Fahrenheit).
To see what effect this heating had on the tumors, the mice were injected with a radioactive tracer incorporated in a molecule similar to glucose, the main energy source in the body. Positron emission and computerized tomography (PET and CT) scans were used to record the concentration of the glucose lookalike in body tissues; the higher the glucose uptake, the greater the metabolic activity.
The tumors of nanocage-injected mice were significantly fainter on the PET scans than those of buffer-injected mice, indicating that many tumor cells were no longer functioning.
The tumors in the nanocage-treated mice were later found to have marked histological signs of cellular damage.
Aktiv inriktning
The scientists have just received a five-year, $2, 129, 873 grant from the National Cancer Institute to continue their work with photothermal therapy.
Despite their results, Xia is dissatisfied with passive targeting. Although the tumors took up enough gold nanocages to give them a black cast, only 6 percent of the injected particles accumulated at the tumor site.
Xia would like that number to be closer to 40 percent so that fewer particles would have to be injected. He plans to attach tailor-made ligands to the nanocages that recognize and lock onto receptors on the surface of the tumor cells.
In addition to designing nanocages that actively target the tumor cells, the team is considering loading the hollow particles with a cancer-fighting drug, so that the tumor would be attacked on two fronts.
But the important achievement, from the point of view of cancer patients, is that any nanocage treatment would be narrowly targeted and thus avoid the side effects patients dread.
The TV and radio character the Lone Ranger used only silver bullets, allegedly to remind himself that life was precious and not to be lightly thrown away. If he still rode today, he might consider swapping silver for gold.